Abstract
Patients of Immune Thrombocytopenia (ITP) are at increased risk of bleeding and the risk increases with decreasing platelet count. Although in patients of severe ITP (platelet < 30x109/L), the risk of bleeding is the highest yet not all patients bleed. If bleeders and non-bleeders in severe ITP can be reliably differentiated, the financial, physical and social burden can be significantly reduced. However, the available investigations have not been able to serve this purpose. Therefore, we included sonoclot; a global test of coagulation, as a tool to assess the risk of bleeding in ITP. Sonoclot coagulation and platelet function analyser uses viscoelastometry to measure coagulation. It provides information on the haemostasis process, including coagulation, fibrin gel formation, fibrinolysis, and is also able to assess platelet function. The sonoclot also has advantage of ability to measure platelet function in severe thrombocytopenia. It is a portable machine, which can be used as bedside tool in emergency out patient department (OPD) or for admitted patients.
The study was conducted at department of internal medicine, postgraduate institute of medical education and research, Chandigarh, India. In this prospective observational study, a total of 80 patients of severe ITP (newly diagnosed) with or without active bleeding were included. Fifty of them had no evidence of any bleeding (Non-Bleeder Group) while thirty of them had features of (WHO grade ≥2) bleeding (Bleeder Group). All these patients were clinically evaluated and samples were collected as per unit protocol for ITP. They were evaluated with Hess test, bleeding time (BT), clotting time (CT), prothrombin time (PT), activated partial thromboplastin time (aPTT), and sonoclot signature analysis. For sonoclot; fresh blood samples were drawn in citrated vaccutainer and analysed by sonoclot coagulation and platelet function analyser made by Sienco, Inc, model SCP1, made in USA. The sonoclot signature assessment gives value for activated clotting time (ACT), clot rate (R1) and platelet function (PF).
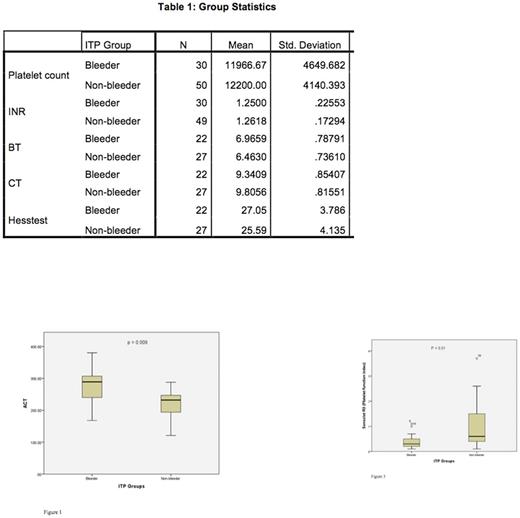
Both the groups were matched in terms of patient age and all had same diagnosis. The mean and standard deviation for variables were as in Table 1. The difference between platelet counts in two groups was non significant (p=.816). The difference between two groups when compared with Hess test (p=.21, INR (p=.794), bleeding time (p=.051) and clotting time (p=.058) was non-significant. However, significant difference in two sonoclot parameters was observed between two groups. ACT was significantly longer in the bleeders compared to non-bleeders (p=.009) and the platelet function measured by sonoclot signature analysis (depicted by R3) was significantly lower in the bleeders than the non-bleeders (p=.01) (Figure 1 and Figure 2). The clot rate (R1) was non-significantly lower in the bleeders.
Although conventional evaluation tools like hess test, bleeding time, clotting time and INR could not differentiate between bleeders and non-bleeders in severe ITP, the coagulation and platelet function parameters assessed by Sonoclot coagulation and platelet function analyser were significantly different between bleeders and non-bleeders. Sonoclot also offers ease of use as compared to other global tests of coagulation (TEG/ ROTEM). Therefore, we conclude that to predict risk of bleeding in severe ITP patients; to help clinician in being more conservative in management of severe ITP patients with low risk of bleeding and avoid unnecessary therapy, sonoclot signature analysis is a handy outpatient or bedside tool.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal